Process intensification
Toward higher efficiency in the manufacturing and quality control of pharmaceutical products
The intensification of manufacturing processes is one of the major challenges facing the pharmaceutical industry. Relying on multidisciplinary and innovative approaches, process intensification offers compact equipement leading to more efficient processes.
For several years, the pharmaceutical authorities have beend promoting the transition toward continuous manufacturing, an ICH Q13 guideline on the continuous manufacturing of drug substances and drug products being currently under redaction. The intensification of processes through the implementation of micro- or milli-structured reactors and the shifting from traditional batch manufacturing to continuous processes, offers more productive and well controlled manufacturing processes.
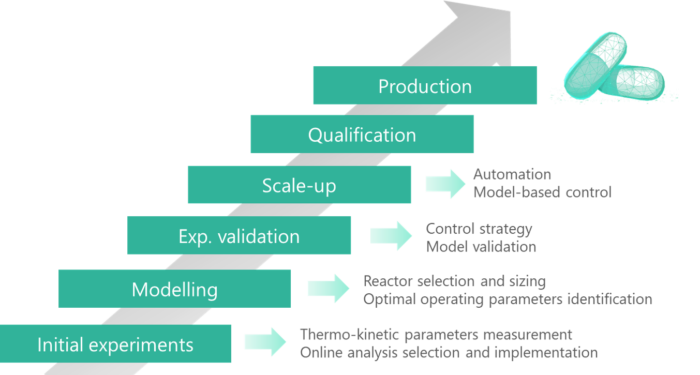
Full characterization of the process since lab scale
Among all its advantages, the possibilities of scale-up via replication of a unit system guarantee the consistency of the product over scale-up and therefore allow a full characterisation of the process at lab scale. It gives the opportunity to design an equipment dedicated for a defined product and specification, hence being in adequacy with the pharmaceutical authorities’ guidance for quality by design. Besides, a better monitoring and control over the critical process parameters and a well understood process will allow a model-based control affording an enhanced product quality consistency.
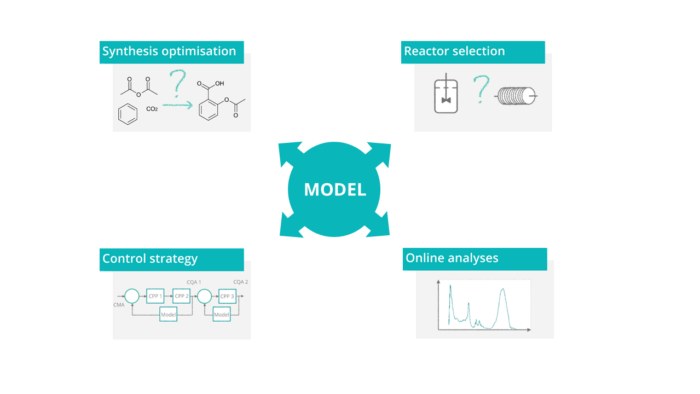
Characteristics
Mechanistic model based control:
- Quality by Design
- Efficient control strategy
- Real time release
- Digital twin level 3 ready
Easier scale-up:
- Reduced development cycles
- Contained danger of scale-up failure
- Opportunities for low-scale development
- Reduced material consumption
Your benefits
Lower reaction volumes:
- Low risks
- Low working capital
- Small process footprint
Higher heat and mass transfer:
- Uniform temperature
- Narrow RTD
- No mass transfer
- Broader range of parameters accessible
- Efficient PAT integration
Better control of the process:
- Higher stability
- Consistent product quality
- Better understanding
Tailored support, every step of the way
SECOYA accompanies its customers throughout the development and implementation of their intensified chemical processes. We intervene throughout every step of the process development, from the early determination of thermokinetic descriptors, in order to build a comprehensive model toward the manufacturing and validation of operational pilot units. These units can then be replicated to achieve the targeted production capacity
Want to know more about the PROCESS INTENSIFICATION?
Want to know more about the PROCESS INTENSIFICATION?
| Thank you for Signing Up |


